This is a demo store. No orders will be fulfilled.
Tris(dimethylamino)phosphine, P(NMe2)3

Product Manager
Sandra Forbes
Tris(dimethylamino)phosphine may serve as a deoxygenation and desulfurization reagent.
Recent Literature
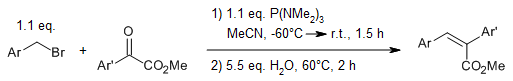
P(NMe₂)₃ facilitates the umpolung alkylation of methyl aroylformates with benzylic and allylic bromides through a two-electron redox addition of the tricoordinate phosphorus reagent to the α-keto ester compound (known as Kukhtin-Ramirez addition). Depending on the post-reaction workup conditions, either Barbier-type addition products or ylide-free olefination products can be obtained.
S. R. Wang, A. T. Radosevich, Org. Lett., 2015, 17, 3810-3813.
DOI: 10.1021/acs.orglett.5b01784
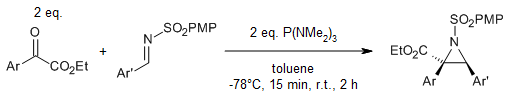
Adducts originating from the reaction of P(NMe₂)₃ and α-ketoesters are efficiently captured by N-sulfonyl imines, yielding a diverse array of aziridine-2-carboxylates. The diastereoselectivity observed in this aziridination process is contingent upon the steric hindrance imposed by substituents present on the substrates.
J. Jiang, H. Liu, C.-D. Lu, Y.-J. Xu, J. Org. Chem., 2017, 82, 811-818.
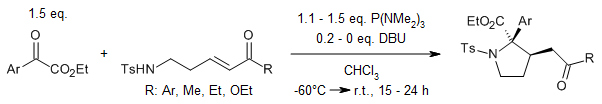
A P(NMe₂)₃-mediated tandem (1 + 4) annulation reaction between aroylformates and δ-tosylamino enones yields functionalized pyrrolidines in good quantities, characterized by exclusive chemoselectivity and high diastereoselectivity. This transformation proceeds via a novel P(NMe₂)₃-mediated reductive amination/base-catalyzed Michael addition cascade mechanism.
R. Liu, J. Liu, J. Cao, R. Li, R. Zhou, Y. Qiao, W.-C. Gao, Org. Lett., 2020, 22, 6922-6926.
DOI: 10.1021/acs.orglett.0c02453
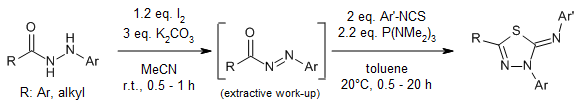
A straightforward P(NMe₂)₃-mediated annulation reaction between N-acyldiazenes and isothiocyanates leads to the formation of 2-imino-1,3,4-thiadiazoles. N-Acyldiazenes can be synthesized from hydrazides utilizing iodine as the oxidizing agent and employed directly without purification.
Z. Huang, Q. Zhang, Q. Zhao, W. Yu, J. Chang, Org. Lett., 2020, 22, 4378-4382.
DOI: 10.1021/acs.orglett.0c01393
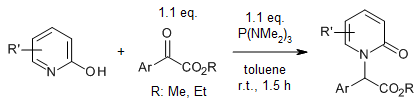
P(NMe₂)₃ facilitates a practical and regioselective direct N-alkylation of 2-pyridones with α-keto esters through a deoxygenation mechanism. This reaction occurs under mild conditions, yielding N-alkylated 2-pyridones with high selectivity and broad applicability. The procedure is scalable for larger-scale synthesis.
N. Wang, Y. Huang, Y. Zi, M. Wang, W. Huang, J. Org. Chem., 2024, 89, 3657-3665.
Quoted from:
https://www.organic-chemistry.org/chemicals/reductions/tris(dimethylamino)phosphine.shtm
Aladdinsci: https://www.aladdinsci.com
