Il s'agit d'un magasin de démonstration. Aucune commande ne sera honorée.
Boronic Acids and Derivatives

Product Manager:Nick Wilde
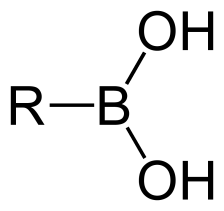
Structurally, boronic acids and derivatives are organic compounds containing trivalent boron, featuring alkyl substituents and hydroxyl groups that satisfy the boron atom’s valence. Due to its electron-deficient nature, the sp²-hybridized boron atom retains an empty p-orbital. This low-energy orbital is orthogonal to its three substituents. Unlike carboxylic acids and their carbon-based analogs, boronic acids do not occur naturally.
Applications
Chemical Use: Boronic acids and derivatives are synthesized from primary boron sources, such as boric acid produced by the acidification of borate with carbon dioxide. Their mild Lewis acidity, combined with stability and ease of handling, makes them valuable synthetic intermediates. Additionally, boronic acids are considered environmentally friendly due to their low toxicity and degradation into boric acid. These properties enable their use in diverse chemical reactions, including Suzuki coupling, Chan–Lam coupling, Liebeskind–Srogl coupling, C–H coupling, and protonolysis. For instance, the borylation of arenes can transform unactivated arene reagents into synthetically versatile products.
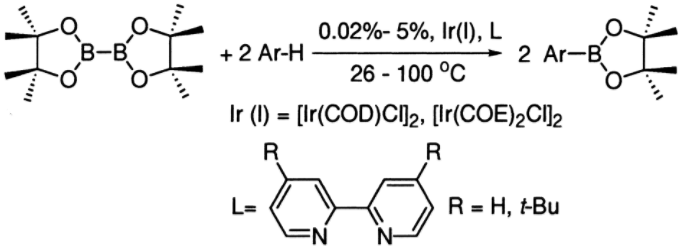
Figure 1. Iridium-catalyzed borylation of arenes
Biological Use: Boronic acids and derivatives are highly useful in biological applications due to their interconvertibility, Lewis acidity, and unique behavior under neutron bombardment. These properties allow their use as enzyme inhibitors, sensors, lectin mimics (boronolectins), boron neutron capture therapy agents, and transmembrane transporters. They are also employed in bioconjugation and immobilization processes.
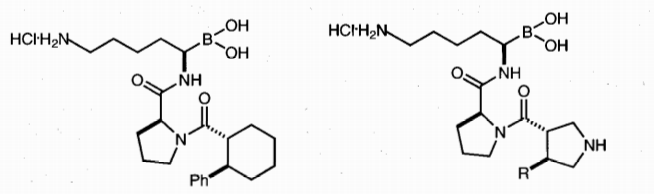
Figure 2. Boronic acid-based thrombin inhibitors
Medicinal Use: The application of boronic acids and derivatives as enzyme inhibitors highlights boron’s therapeutic potential. For example, Bortezomib, a boronic acid-based inhibitor, has been FDA-approved for treating multiple myeloma.
Reference
1. Ishiyama T, Takagi J, Ishida K, et al. Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate[J]. Journal of the American Chemical Society, 2002, 124(3): 390-391.
Aladdin:https://www.aladdinsci.com
