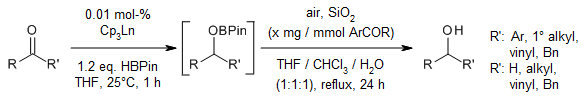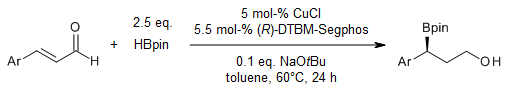This is a demo store. No orders will be fulfilled.
Pinacolborane
Product Manager
Sandra Forbes


Recent Literature
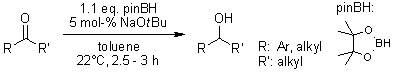
The reduction of ketones employing pinacolborane is accelerated by NaOt-Bu at room temperature. This reaction yields substantial amounts of product and is generalizable, achieving complete conversion of aryl and dialkyl ketones. The active hydride source is trialkoxyborohydride, which is believed to be present at low concentrations under the reaction conditions.
I. P. Query, P. A. Squier, E. M. Larson, N. A. Isley, T. B. Clark, J. Org. Chem., 2011, 76, 6452-6456.
DOI: 10.1021/jo201142g
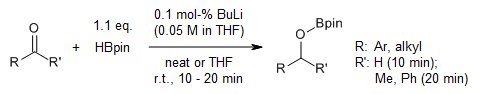
Small amounts of n-BuLi catalyze an efficient and selective hydroboration of aldehydes and ketones with HBpin. The reaction occurs swiftly under mild conditions, demonstrating exceptional functional group compatibility, a broad substrate range, and high selectivity for aldehydes over ketones.
Z. Zhu, X. Wu, X. Xu, Z. Wu, M. Xue, Y. Yao, Q. Shen, X. Bao, J. Org. Chem., 2018, 83, 10677-10683.
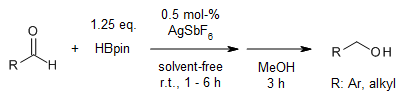
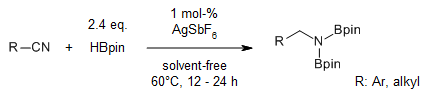
Low catalyst loadings of AgSbF6 catalyze the hydroboration of nitriles, alkenes, and aldehydes under base- and solvent-free conditions. This atom-economic, chemoselective protocol exhibits excellent functional group tolerance and compatibility with substrates that vary structurally and electronically.
V. K. Pandey, C. S. Tiwari, A. Rit, Org. Lett., 2021, 23, 1681-1686.
DOI: 10.1021/acs.orglett.1c00106
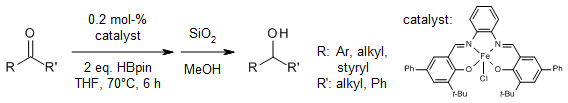
Iron(III) salen complexes catalyze selective hydroborations of ketones and unactivated imines with pinacolborane in the absence of any additives. These catalyst systems yield significant amounts of product, demonstrate chemoselectivity, high atom economy, and a broad substrate range under mild reaction conditions with minimal catalyst loading. This synthesis of alcohols can be easily scaled up to the gram scale.
A. T. Latha, C. A. Swamy P, J. Org. Chem., 2024, 89, 8376-8384.
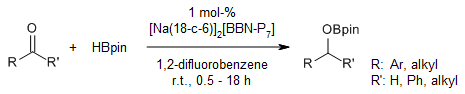
A boron-functionalized heptaphosphide Zintl cluster catalyzes a transition metal-free hydroboration of aldehydes and ketones. Additionally, the greenhouse gas carbon dioxide is efficiently and selectively reduced to methoxyborane.
B. van IJzendoorn, S. F. Albawardi, I. J. Votorica-Yrezabal, G. F. S. Whitehead, J. E. McGrady, M. Mehta, J. Am. Chem. Soc., 2022, 144, 21213-21223.
DOI: 10.1021/jacs.2c08559
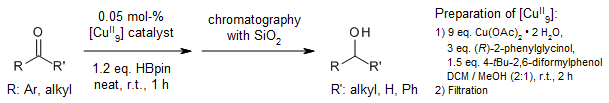
A nonanuclear copper(II) complex obtained through a facile one-pot self-assembly catalyzes the hydroboration of ketones and aldehydes in the absence of an activator under mild, solvent-free conditions. This air- and moisture-stable catalyst displays high efficiency and chemoselectivity, favoring aldehydes over ketones and ketones over imines.
H. Zeng, J. Wu, S. Li, C. Hui, A. Ta, S.-Y. Cheng, S. Zheng, G. Zhang, Org. Lett., 2019, 21, 401-406.
DOI: 10.1021/acs.orglett.8b03583
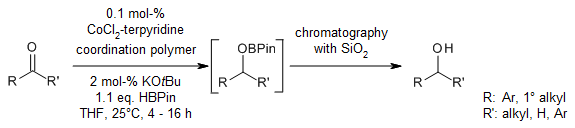
A recyclable cobalt(II)-terpyridine coordination polymer (CP) serves as a highly effective hydroboration precatalyst for the reductions of ketones, aldehydes, and imines with pinacolborane (HBpin). A wide range of substrates containing polar C=O or C=N bonds undergo selective hydroboration in excellent yields under ambient conditions.
J. Wu, H. Zeng, J. Cheng, S. Zheng, J. A. Golen, D. R. Manke, G. Zhang, J. Org. Chem., 2018, 83, 9442-9448.
Homoleptic cyclopentadienyl lanthanide complexes are excellent catalysts for the hydroboration of various aldehydes and ketones with pinacolborane. These robust lanthanide catalysts exhibit high reactivity with low catalyst loadings under mild conditions, good functional group tolerance, and unique carbonyl-selectivity.
S. Chen, D. Yan, M. Xue, Y. Hong, Y. Yao, Q. Shen, Org. Lett., 2017, 19, 3382-3385.
DOI: 10.1021/acs.orglett.7b01335
Chemoselective Luche-Type Reduction of α,β-Unsaturated Ketones via Magnesium Catalysis
Y. K. Jang, M. Magre, M. Rueping, Org. Lett., 2019, 21, 8349-8352.
DOI: 10.1021/acs.orglett.9b03131
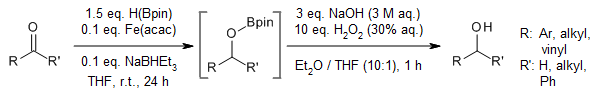
An operationally convenient hydroboration of aldehydes and ketones using Fe(acac)3 as a precatalyst proceeds efficiently at room temperature, yielding 1° and 2° alcohols after workup. A σ-bond metathesis mechanism involving an Fe-H intermediate as the key reactive species is proposed.
S. R. Tamang, M. Findlater, J. Org. Chem., 2017, 82, 12857-12862.
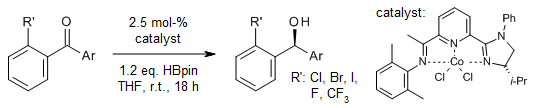
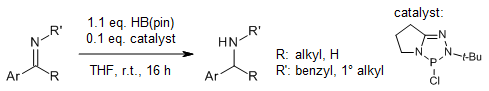
A chiral imidazole iminopyridine ligand enables highly enantioselective cobalt-catalyzed hydroboration of diaryl ketones with pinacolborane, providing chiral benzhydrols in very good yields and ee. This protocol can be conducted on a gram scale under mild reaction conditions with good functional group tolerance.
W. Liu, J. Guo, S. Xing, Z. Lu, Org. Lett., 2020, 22, 2532-2536.
DOI: 10.1021/acs.orglett.0c00293
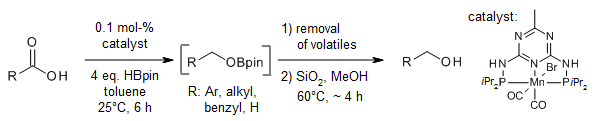
A manganese-catalyzed chemoselective hydroboration of carboxylic acids to the corresponding alcohols offers a high turnover number and frequency at 25°C. This method tolerates electronically and sterically differentiated substrates with high chemoselectivity. Importantly, aliphatic long-chain fatty acids, including biomass-derived compounds, can be efficiently reduced.
M. K. Barman, K. Das, B. Maji, J. Org. Chem., 2019, 84, 1570-1579.
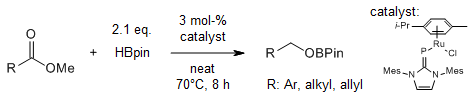
The ruthenium complex [(η6-p-cymene){(IMes)P}RuCl] is utilized for efficient hydroborations of a wide range of nitriles, carboxylic esters, and carboxamides in neat pinacolborane (HBpin) under relatively mild reaction conditions (60-80 °C, 3-5 mol % catalyst loading).
J. Bhattacharjee, D. Blockfeld, M. Tamm, J. Org. Chem., 2022, 87, 1098-1109.
Organoborane reductants have been made catalytic through isodesmic B-O/B-H transborylation, applied in borane-catalyzed, chemoselective alkene reduction and formal hydrofunctionalization of enones. The reaction proceeds via a 1,4-hydroboration of the enone and B-O/B-H transborylation with HBpin, enabling catalyst turnover.
K. Nicholson, T. Langer, S. P. Thomas, Org. Lett., 2021, 23, 2498-2504.
DOI: 10.1021/acs.orglett.1c00446
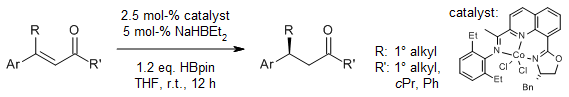
In an operationally simple protocol, an 8-OIQ cobalt complex catalyzes a chemo- and enantioselective 1,4-hydroboration of enones with HBpin, providing access to a broad range of chiral β,β-disubstituted ketones with excellent chemo- and enantioselectivities.
X. Ren, Z. Lu, Org. Lett., 2021, 23, 8370-8374.
DOI: 10.1021/acs.orglett.1c03110
A ruthenium-catalyzed hydroboration of ynones yields vinyl α-hydroxylboronates under mild conditions. This reaction features high efficiency, a broad scope, and complete chemo-, regio-, and stereoselectivity, despite numerous possible competitive pathways.
Q. Feng, S. Li, Z. Li, Q. Yan, X. Lin, L. Song, X. Zhang, Y.-D. Wu, J. Sun, J. Am. Chem. Soc., 2022, 144, 14846-14855.
DOI: 10.1021/jacs.2c06024
A combination of KOH and BEt3 catalyzes a deaminative hydroboration of acyl-iminodibenzyl derivatives, including nonheterocyclic carboxamides, to the corresponding amines. This novel transition-metal-free methodology is also applied to the hydroboration/reduction of aldehydes.
W. Yao, J. Wang, A. Zhong, J. Li, J. Yang, Org. Lett., 2020, 22, 8086-8090.
DOI: 10.1021/acs.orglett.0c03033
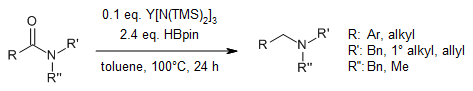
Y[N(TMS)2]3 serves as an efficient homogeneous catalyst for the hydroboration of secondary and tertiary amides to provide the corresponding amines. The reaction tolerates various functional groups, such as cyano, nitro, and vinyl groups.
P. Ye, Y. Shao, X. Ye, F. Zhang, R. Li, J. Sun, B. Xu, J. Chen, Org. Lett., 2020, 22, 1265-1269.
DOI: 10.1021/acs.orglett.9b04606
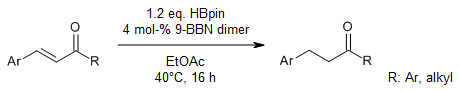
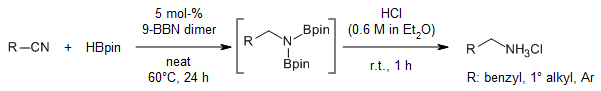
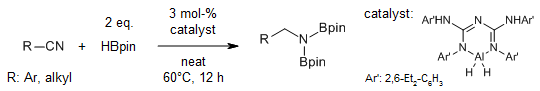
The use of 9-BBN dimer as a catalyst and pinacolborane as a turnover reagent enables an efficient hydroboration of nitriles to yield N,N-diborylamines, which act as efficient synthons for the synthesis of primary amines and secondary amides.
S. Pradhan, R. V. Sankar, C. Gunanathan, J. Org. Chem., 2022, 87, 12386-12396.
Low catalyst loadings of AgSbF6 catalyze the hydroboration of nitriles, alkenes, and aldehydes under base- and solvent-free conditions. This atom-economic, chemoselective protocol exhibits excellent functional group tolerance and compatibility with substrates that vary structurally and electronically.
V. K. Pandey, C. S. Tiwari, A. Rit, Org. Lett., 2021, 23, 1681-1686.
DOI: 10.1021/acs.orglett.1c00106
The ruthenium complex [(η6-p-cymene){(IMes)P}RuCl] is utilized for efficient hydroborations of a wide range of nitriles, carboxylic esters, and carboxamides in neat pinacolborane (HBpin) under relatively mild reaction conditions (60-80 °C, 3-5 mol % catalyst loading).
J. Bhattacharjee, D. Blockfeld, M. Tamm, J. Org. Chem., 2022, 87, 1098-1109.
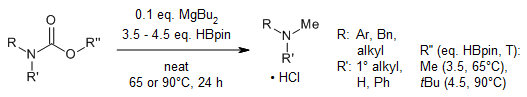
A magnesium-catalyzed reduction of linear and cyclic carbamates, including N-Boc protected amines, yields N-methyl amines and amino alcohols of significant interest due to their presence in many biologically active molecules. Furthermore, the reduction can be extended to the formation of N-trideuteromethyl-labeled amines.
M. Magre, M. Szewczyk, M. Rueping, Org. Lett., 2020, 22, 3209-3214.
DOI: 10.1021/acs.orglett.0c00988
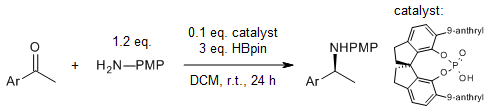
SPINOL-derived borophosphates catalyze an asymmetric reductive amination of ketones with pinacolborane to yield a series of chiral amine derivatives bearing multiple functional groups in very good yields and enantioselectivities under mild reaction conditions.
Z. Wu, H. He, M. Chen, L. Zhu, W. Zheng, Y. Cao, J. C. Antilla, Org. Lett., 2022, 24, 9436-9441.
DOI: 10.1021/acs.orglett.2c03866
Cobalt chloride catalyzes a borylative reduction of azobenzenes using pinacolborane to yield hydrazobenzenes. This borylative reduction demonstrates good functional group compatibility and can be readily scaled up to the gram scale.
W. Wang, Y. Wang, Y. Yang, S. Xie, Q. Wang, W. Chen, S. Wang, F. Zhang, Y. Shao, J. Org. Chem., 2024, 89, 9265-9274.
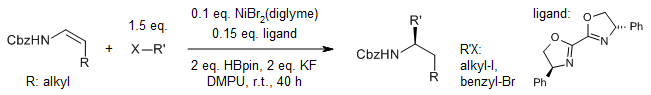
A nickel-catalyzed hydroalkylation of enecarbamates (N-Cbz-protected enamines) with alkyl halides yields a wide range of chiral alkyl amines with high regio- and enantioselectivity. The mild conditions lead to high functional group tolerance, demonstrated in late-stage modifications of many natural products and drug molecules.
D. Qian, S. Bera, X. Hu, J. Am. Chem. Soc., 2021, 143, 1959-1967.
DOI: 10.1021/jacs.0c11630
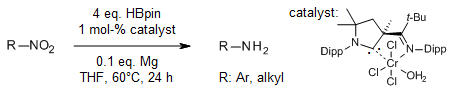
A complex of a cyclic (alkyl)(amino)carbene (CAAC) ligand with chromium catalyzes a mild, chemoselective, and efficient deoxygenative hydroboration of nitro compounds to yield a broad range of anilines, as well as heteroaryl and aliphatic amine derivatives. The CAAC ligand plays a crucial role in promoting polarity reversal of the hydride of HBpin and serves as an H-shuttle.
L. Zhao, C. Hu, X. Cong, G. Deng, L. L. Liu, M. Luo, X. Zeng, J. Am. Chem. Soc., 2021, 143, 1618-1629.
DOI: 10.1021/jacs.0c12318
A chiral phosphoric acid catalyzes an asymmetric transfer hydrogenation of trans-chalcones in the presence of pinacolborane as a hydride source. This methodology yields chiral dihydrochalcone derivatives in high yields and with high enantioselectivities under mild conditions.
F. Na, S. S. Lopez, A. Beauseigneur, L. W. Hernandez, Z. Sun, J. C. Antilla, Org. Lett., 2020, 22, 5953-5957.
DOI: 10.1021/acs.orglett.0c02042
A copper-catalyzed enantioselective hydroboration of α,β-unsaturated aldehydes with pinacolborane yields the corresponding γ-pinacolboronate alcohols in good yields and enantioselectivities through consecutive hydroboration of the C=O and C=C bonds. The resulting γ-pinacolboronate alcohols can be utilized in various transformations.
W. J. Jang, S. M. Song, Y. Park, J. Yun, J. Org. Chem., 2019, 84, 4429-4434.
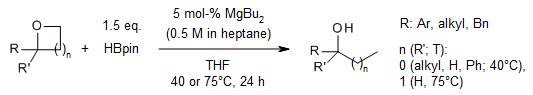
A readily available magnesium catalyst achieves a selective hydroboration of a wide range of epoxides and oxetanes, yielding secondary and tertiary alcohols in excellent yields and regioselectivities.
M. Magre, E. Paffenholz, B. Maity, L. Cavallo, M. Rueping, J. Am. Chem. Soc., 2020, 142, 14286-14294.
DOI: 10.1021/jacs.0c05917
A conjugated bis-guanidinate (CBG)-supported aluminum dihydride complex catalyzes a chemoselective hydroboration of various nitriles and alkynes. The reaction leaves other reducible groups intact. Furthermore, aluminum-catalyzed hydroboration is extended to more challenging substrates such as alkenes, pyridines, imines, carbodiimides, and isocyanides.
N. Sarkar, S. Bera, S. Nembenna, J. Org. Chem., 2020, 85, 4999-5009.
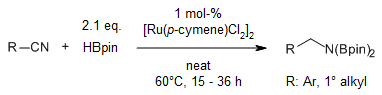
A simple [Ru(p-cymene)Cl2]2 complex is employed as a catalyst precursor in a catalyzed hydroboration of nitriles and imines using pinacolborane with unprecedented catalytic efficiency.
A. Kaithal, B. Chatterjee, C. Gunanathan, J. Org. Chem., 2016, 81, 11153-11161.
1,2,4,3-Triazaphospholene halides catalyze the 1,2-hydroboration of imines and α,β-unsaturated aldehydes with pinacolborane, including examples that did not undergo hydroboration with previously reported diazaphospholene systems. DFT calculations support a mechanism where a triazaphospholene cation interacts with the substrate.
C.-H. Tien, M. R. Adams, M. J. Ferguson, E. R. Johnson, A. W. H. Speed, Org. Lett., 2017, 19, 5565-5568.
DOI: 10.1021/acs.orglett.7b02695
A simple [Ru(p-cymene)Cl2]2 complex is employed as a catalyst precursor in a catalyzed hydroboration of nitriles and imines using pinacolborane with unprecedented catalytic efficiency.
A. Kaithal, B. Chatterjee, C. Gunanathan, J. Org. Chem., 2016, 81, 11153-11161.
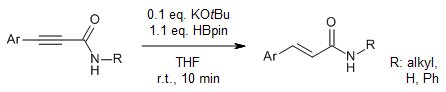
A transition-metal-free semireduction of 3-substituted primary and secondary propiolamides with pinacolborane and catalytic potassium tert-butoxide yields 3-substituted acrylamide derivatives in very good yield with excellent E selectivity. Mechanistic studies suggest that an activated Lewis acid-base complex transfers a hydride to the α-carbon, followed by rapid protonation in a trans fashion.
R. J. Grams, C. J. Garcia, C. Szwetkowski, W. L. Santos, Org. Lett., 2020, 22, 7013-7018.
DOI: 10.1021/acs.orglett.0c02567
A reductive three-component coupling of terminal alkynes, aryl halides, and pinacolborane yields benzylic alkyl boronates in good yields via a hydrofunctionalization of both π-bonds of the alkyne promoted by cooperative action of the catalysts. The reaction offers an excellent substrate range and tolerates the presence of esters, nitriles, alkyl halides, epoxides, acetals, and alkenes.
M. K. Armstrong, G. Lalic, J. Am. Chem. Soc., 2019, 141, 6173-6179.
DOI: 10.1021/jacs.9b02372
Copper-Catalyzed Reductive Ireland-Claisen Rearrangements of Propargylic Acrylates and Allylic Allenoates
S. Guo, K. C. Wong, S. Scheeff, Z. He, W. T. K. Chan, K.-H. Low, P. Chiu, J. Org. Chem., 2022, 87, 429-452.
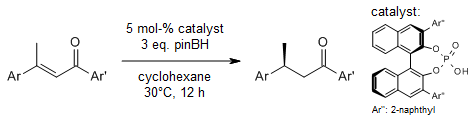
A chiral boro-phosphate catalyzes a reductive amination of 2,2-disubstituted 1,3-cyclopentadiones with pinacolborane as the reducing agent to yield chiral β-amino ketones with an all-carbon quaternary stereocenter in good yields, high enantioselectivities, and excellent diastereoselectivities. This desymmetrization reaction has a broad substrate range and high functional group tolerance.
M. Chen, L. Zhu, W. Zheng, Y. Fu, J. Zhang, H. He, J. C. Antilla, Org. Lett., 2024, 26, 3951-3956.
DOI: 10.1021/acs.orglett.4c01195
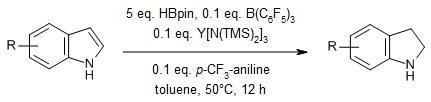
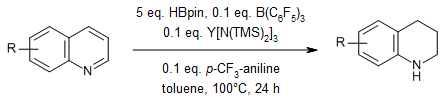
A lanthanide/B(C6F5)3-promoted hydroboration reduction of indoles and quinolines with pinacolborane (HBpin) yields a range of nitrogen-containing compounds in good yields. Large-scale synthesis and further transformations to bioactive compounds indicate that the method has potential practical applications.
J. Zhang, Z. Chen, M. Chen, Q. Zhou, R. Zhou, W. Wang, Y. Shao, F. Zhang, J. Org. Chem., 2024, 89, 887-897.
A lanthanide/B(C6F5)3-promoted hydroboration reduction of indoles and quinolines with pinacolborane (HBpin) yields a range of nitrogen-containing compounds in good yields. Large-scale synthesis and further transformations to bioactive compounds indicate that the method has potential practical applications.
J. Zhang, Z. Chen, M. Chen, Q. Zhou, R. Zhou, W. Wang, Y. Shao, F. Zhang, J. Org. Chem., 2024, 89, 887-897.
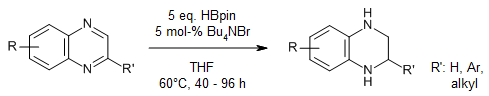
The use of HBpin as a hydrogen source enables a metal-free, environmentally benign, simple, and efficient transfer hydrogenation process of quinoxalines to yield the desired tetrahydroquinoxalines in good yields in the presence of Bu4NBr as a noncorrosive and low-cost catalyst.
Q. Guo, J. Chen, G. Shen, G. Lu, X. Yang, Y. Tang, Y. Zhu, S. Wu, B. Fan, J. Org. Chem., 2022, 87, 540-546.
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/pinacolborane.shtm
Aladdinsci: https://www.aladdinsci.com