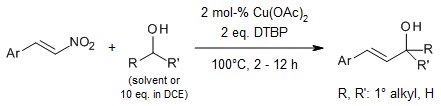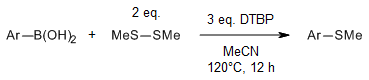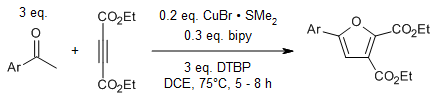This is a demo store. No orders will be fulfilled.
Di-tert-butyl peroxide (DTBP)
Product Manager:NickWilde


Di-tert-butyl peroxide is a highly stable organic peroxide frequently utilized as a radical initiator due to its ability to undergo homolysis at temperatures exceeding 100°C.
Recent Literature

Novel ionic iron(III) complexes, featuring an imidazolinium cation, catalyze the esterification of primary benzylic C-H bonds with carboxylic acids, utilizing di-tert-butyl peroxide as the oxidant. This reaction exhibits broad applicability and accommodates sterically hindered reactants.
B. Lu, F. Zhu, H.-M. Sun, Q. Shen, Org. Lett., 2017, 19, 1132-1135.
https://doi.org/10.1021/acs.orglett.7b00148

When a catalytic quantity of copper(II) acetate and di-tert-butyl peroxide is present, anilines undergo a cross-coupling reaction with alkylborane reagents, resulting in the production of N-alkylated anilines with yields ranging from good to excellent. Additionally, phenols can also be utilized in this reaction.
S. Sueki, Y. Kuninobu, Org. Lett., 2013, 15, 1544-1547.
https://doi.org/10.1021/ol400323z

The success of a general catalytic procedure for cross-coupling primary amides with alkylboronic acids hinged on discovering a gentle base (NaOSiMe3) and an oxidant (di-tert-butyl peroxide) that could enhance the copper-catalyzed reaction, leading to high yields. This method offers a straightforward and efficient way to achieve monoalkylation of amides.
S. A. Rossi, K. W. Shimkin, Q. Xu, L. M. Mori-Quiroz, D. A. Watson, Org. Lett., 2013, 15, 2314-2317.
https://doi.org/10.1021/ol401004r

A copper-catalyzed amidation reaction between benzylic hydrocarbons and inert aliphatic alkanes, using simple amides, occurred smoothly without the need for a ligand. This process allowed for the synthesis of a diverse array of N-alkylated aromatic and aliphatic amides, sulfonamides, and imides in satisfactory yields.
H.-T. Zeng, J.-M. Huang, Org. Lett., 2015, 17, 4276-4279.
https://doi.org/10.1021/acs.orglett.5b02063

An abundant iron catalyst facilitates the dehydrogenative acylation of enamides with aldehydes, yielding valuable β-ketoenamides that exhibit exceptional tolerance to functional groups. This C-H acylation process occurs with complete Z-selectivity.
R.-H. Liu, Z.-Y. Shen, C. Wang, T.-P. Loh, X.-H. Hu, Org. Lett., 2020, 22, 944-949.
https://doi.org/10.1021/acs.orglett.9b04495

A straightforward Fe-catalyzed process enables dual-functionalization and cross-coupling of two distinct alkenes, resulting in chain-elongated aromatic alkenes that are trifluoromethylated and follow an A-D-A-T-type pattern.
J. Zhao, R.-X. Liu, C.-P. Luo, L. Yang, Org. Lett., 2020, 22, 6776-6779.
https://doi.org/10.1021/acs.orglett.0c02267
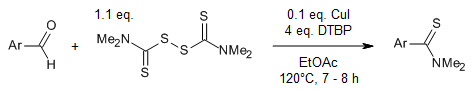
A straightforward approach for synthesizing aryl thioamides from aryl aldehydes and tetramethylthiuram disulfide (TMTD), utilizing CuI and di-tert-butyl peroxide (DTBP), eliminates the need for a sulfurating reagent. This method boasts a wide range of substrates, excellent yields, practical operability, and employs readily available and cost-effective raw materials.
M.-T. Zeng, M. Wang, H.-Y. Peng, Y. Cheng, Z.-B. Dong, Synthesis, 2018, 50, 644-650.
https://doi.org/10.1055/s-0036-1590936
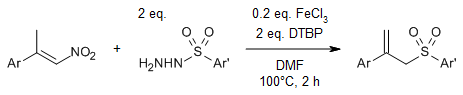
Regiospecific radical reactions of β-alkyl nitroalkenes with sulfonyl hydrazides provide allyl sulfones with high regioselectivity in the presence of dimethylformamide (DMF), whereas reactions in acetonitrile provide vinyl sulfones.
Y. Wang, G. Xiong, C. Zhang, Y. Chen, J. Org. Chem., 2021, 86, 4018-4026.
https://doi.org/10.1021/acs.joc.0c02869
Utilizing di-tert-butyl peroxide as a radical initiator in conjunction with a copper salt as a promoter facilitates the synthesis of allylic alcohol, benzyl, and alkane derivatives through a radical mechanism. The C(sp3)-H bonds present in various alcohols, toluene derivatives, and alkanes were successfully alkenylated with β-nitrostyrenes, resulting in the desired products with high yields.
S.-r. Guo, Y.-q. Yuan, Synlett, 2015, 26, 1961-1968.
https://doi.org/10.1055/s-0034-1380445
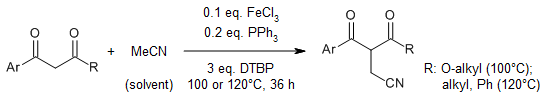
An effective approach allows for the production of α-cyanomethyl-β-dicarbonyls in high yields, starting from MeCN and straightforward 1,3-dicarbonyls. This process is hypothesized to operate through a radical mechanism.
C. Wang, Y. Li, M. Gong, Q. Wu, J. Zhang, J. K. Kim, M. Huang, Y. Wu, Org. Lett., 2016, 18, 4151-4153.
https://doi.org/10.1021/acs.orglett.6b01871
A metal-free coupling reaction between arylboronic acids and dimethyldisulfide efficiently produces aryl methyl sulfides. This method is straightforward, yields satisfactory results, demonstrates high functional-group compatibility, and operates under mild conditions.
X.-m. Wu, J.-m. Lou, G.-b. Yan, Synlett, 2016, 27, 2269-2273.
https://doi.org/10.1055/s-0035-1562499
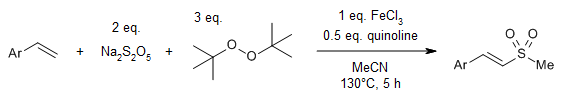
Employing inorganic sodium metabisulfite as a sulfur dioxide surrogate and di-tert-butyl peroxide as a source of methyl radicals facilitates a direct C-H methylsulfonylation of alkenes. This approach offers a straightforward route to (E)-2-methyl styrenyl sulfones with good yields.
F.-S. He, Y. Gong, P. Rojsitthisak, J. Wu, J. Org. Chem., 2019, 84, 13159-13163.
https://doi.org/10.1021/acs.joc.9b01729
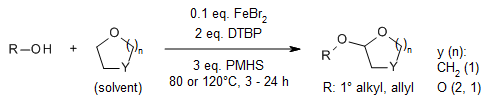
An iron-catalyzed α-C(sp3)-H activation of cyclic and acyclic ethers provides an efficient and green method for the synthesis of mixed acetals in very good yields. The robustness of this protocol is demonstrated by the late-stage oxidation of a structurally complex natural product.
W. Han, L. Cheng, H. Zhao, Synlett, 2020, 31, 1400-1403.
https://doi.org/10.1055/s-0040-1707162
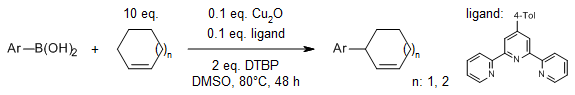
Cu2O, which is inexpensive, abundant, and nontoxic, catalyzes a regioselective oxidative allylic C(sp3)-H arylation of easily accessible unactivated terminal and internal olefins using a wide variety of heteroaryl boronic acids. This approach avoids the need for conventional coupling partners with pre-installed leaving groups at the allylic position, thereby providing an alternative route to allylic arylation.
S. Pal, M. Cotard, B. Gérardin, C. Hoarau, C. Schneider, Org. Lett., 2021, 23, 3130-3135.
https://doi.org/10.1021/acs.orglett.1c00812
A synthesis of multisubstituted furans can be achieved using Copper(I) salts as catalysts, starting from readily accessible acetophenones and electron-deficient alkynes. This process involves direct C(sp3)-H bond functionalization under radical reaction conditions, with di-tert-butyl peroxide serving as an external oxidant. This method provides an efficient route to biologically significant scaffolds from simple precursors.
S. Manna, A. P. Antonchick, Org. Lett., 2015, 17, 4300-4303.
https://doi.org/10.1021/acs.orglett.5b02114
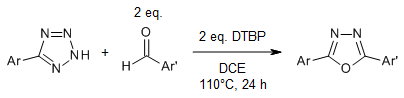
A metal- and base-free one-pot synthesis of 2,5-diaryl 1,3,4-oxadiazoles can be achieved through a radical-promoted cross-dehydrogenative coupling strategy. This involves N-acylation of aryl tetrazoles with aryl aldehydes, followed by thermal rearrangement. A wide variety of aryl tetrazoles and aryl aldehydes can be used to produce the corresponding products in good yields.
L. Wang, J. Cao, Q. Chen, M. He, J. Org. Chem., 2015, 80, 4743-4748.
https://doi.org/10.1021/acs.joc.5b00207
A highly regioselective and versatile synthesis of acylated N-heterocycles can be achieved through cascade reactions of saturated cyclic amines with 2-oxo-2-arylacetic acids. In this cascade process, the copper catalyst plays a pivotal role, facilitating not only dehydrogenation but also decarboxylation and cross-coupling reactions.
X. Shi, X. Chen, M. Wang, X. Zhang, X. Fan, J. Org. Chem., 2018, 83, 6524-6533.
https://doi.org/10.1021/acs.joc.8b00805
A copper-catalyzed sp3 C-H functionalization of 2-alkyl-N-substituted benzamides offers an effective method for synthesizing various functionalized isoindolinones, without the need for halogenated substitutes, expensive transition metals, or toxic Sn or CO gas.
K. Nozawa-Kumada, J. Kadokawa, T. Kameyama, Y. Kondo, Org. Lett., 2015, 17, 4479-4481.
https://doi.org/10.1021/acs.orglett.5b02235
An iodine-mediated oxidative intramolecular amination of anilines, which does not require a transition metal catalyst, produces indolines through the cleavage of unactivated (sp3)C-H and N-H bonds. This reaction can be scaled up to the gram level for the synthesis of functionalized indolines.
J. Long, X. Cao, L. Zhu, R. Qiu, C.-T. Au, S.-F. Yin, T. Iwasaki, N. Kambe, Org. Lett., 2017, 19, 2793-2796.
https://doi.org/10.1021/acs.orglett.7b00846
Substituted thiophenols can undergo iodine-catalyzed cascade reactions with alkynes under metal- and solvent-free conditions, enabling the synthesis of benzothiophene derivatives in good yields. This efficient, economical, and environmentally friendly transformation offers an appealing route to various benzothiophenes.
K. Yan, S. Yang, M. Zhang, W. Wei, Y. Liu, L. Tian, H. Wang, Synlett, 2015, 26, 1890-1894.
https://doi.org/10.1055/s-0034-1378841
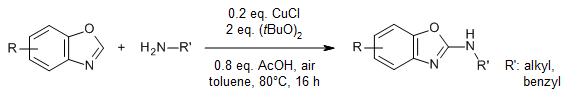
Copper-catalyzed C-H bond activation enables a facile, efficient, and simple protocol for direct oxidative C-H amination of benzoxazoles with primary amines using di-tert-butyl peroxide (DTBP) as oxidant under air. Various substituted aminobenzoxazoles were synthesized with very good yields.
J. Gu, C. Cai, Synlett, 2015, 26, 639-642.
https://doi.org/10.1055/s-0034-1379886
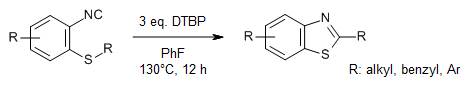
Triggered by alkyl radicals, a wide range of 2-isocyanoaryl thioethers, which include aliphatic, aryl, and heteroaromatic groups, can be cleaved and reinstalled precisely to produce benzothiazole derivatives. Mechanistic studies indicate that the cascade reaction follows an intermolecular pathway.
K. Luo, W.-C. Yang, K. Wei, Y. Liu, J.-K. Wang, L. Wu, Org. Lett., 2019, 21, 7851-7856.
https://doi.org/10.1021/acs.orglett.9b02837
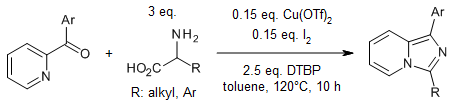
Using a copper/iodine cocatalyst system, the decarboxylative cyclization of α-amino acids with either 2-benzoylpyridines or 2-benzoylquinolines yields 1,3-disubstituted imidazo[1,5-a]pyridines and 1,3-disubstituted imidazo[1,5-a]quinolines in high yields.
H. Wang, W. Xu, L. Xin, W. Liu, Z. Wang, K. Xu, J. Org. Chem., 2016, 81, 3681-3687.
https://doi.org/10.1021/acs.joc.6b00343
Pharmaceutically significant azolo[1,5-a]pyrimidines can be synthesized through the use of readily available 3- or 5-aminoazoles, aldehydes, and triethylamine. The crucial step involves the in situ formation of an acyclic enamine, followed by an annulation reaction. This method allows for the synthesis of various 5,6-unsubstituted pyrazolo[1,5-a]pyrimidines and [1,2,4]triazolo[1,5-a]pyrimidines.
Q. Gao, Z. Sun, Q. Xia, R. Li, W. Wang, S. Ma, Y. Chai, M. Wu, W. Hu, P. Ábrányi-Baloghm G. M. Keserű, X. Han, Org. Lett., 2021, 23, 2621-2625.
https://doi.org/10.1021/acs.orglett.1c00571
An iron(III)-catalyzed oxidative coupling reaction between alcohols or methyl arenes and 2-amino phenyl ketones can synthesize a wide variety of 4-quinolones. In the presence of an iron catalyst and di-tert-butyl peroxide, the alcohols or methyl arenes are first oxidized to aldehydes, which then undergo condensation with the amine, followed by a Mannich-type cyclization and oxidation.
S. B. Lee, Y. Jang, J. Ahn, S. Chun, D.-C. Oh, S. Hong, Org. Lett., 2020, 22, 8382-8386.
https://doi.org/10.1021/acs.orglett.0c03011
By making a simple adjustment to the reaction conditions, it becomes possible to efficiently synthesize various valuable 3-aryl- and 3-aroylcoumarins through direct arylation and aroylation of coumarins with glyoxals, all without the need for a metal catalyst. This method supports a wide range of starting materials and produces high yields of both types of cross-coupling reactions starting from the same initial materials.
A. Moazzam, M. Khodadadi, F. Jafarpour, M. Ghandi, J. Org. Chem., 2022, 87, 3630-3637.
https://doi.org/10.1021/acs.joc.1c02159
Cyclic amides can undergo a copper-catalyzed direct amination in dimethylformamide (DMF) to produce aromatic heterocyclic amines using readily available reagents, and this process operates through a radical mechanism. The N1 atom's coordinating effect aids the copper ions in activating and aminating the C-O bonds.
P. Chen, K. Luo, X. Yu, X. Yuan, X. Liu, J. Lin, Y. Jin, Org. Lett., 2020, 22, 6547-6551.
https://doi.org/10.1021/acs.orglett.0c02320
Quoted from: https://www.organic-chemistry.org/chemicals/oxidations/di-tert-butylperoxide-dtbp.shtm
Aladdin:https://www.aladdinsci.com